COVID-19 Antigen(Ag) Rapid Test Device(Cassette with Buffer)
For professional in vitro diagnostic use only.
Intended Use: COVID-19 Ag Rapid Test Device is a rapid chromatographic immunoassay for the qualitative detection of specific antigens to SARS-CoV-2 present in human naso-pharynx. This test is for professional used only, as an aid to early diagnosis of SARS-CoV-2 infection in patient. The result of this test should not be the sole basis for the diagnosis; confirmatory testing is required.
Summary
Coronavirus is a single-stranded positive-sense RNA virus with an envelope of about 80 to 120 nm in diameter. Its genetic material is the largest of all RNA viruses and is an important pathogen of many domestic animals, pets, and human diseases. It can cause a variety of acute and chronic diseases. Common signs of a person infected with a coronavirus include respiratory symptoms, fever, cough, shortness of breath, and dyspnea. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and even death. The 2019 new coronavirus, or “SARS-CoV-2 (COVID-19)” named by the World Health Organization can cause pneumonia epidemic. The detection results of this kit are for clinical reference only. The result of this test should not be the sole basis for the diagnosis; confirmatory testing is required.
Principle
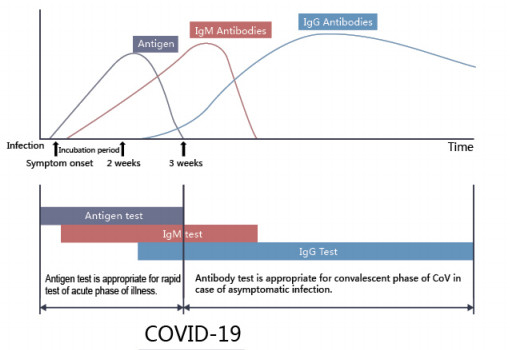
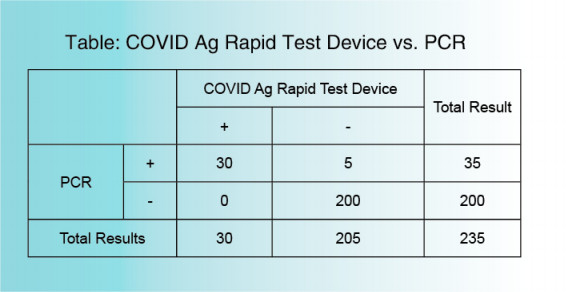
The COVID-19 Ag Rapid Test Device uses double antibody sandwich immunoassay. The NC membrane pre-immobilized with monoclonal antibodies against SARS-CoV-2 antigen and anti-mouse polyclonal antibodies, and the colloidal-gold conjugated with monoclonal antibodies specific to SARS-CoV-2 antigen. If SARS-CoV-2 antigen present in the sample, a complex formed between the anti-SARS-CoV-2 conjugate and the antigen will be caught by the specific anti- SARS-CoV-2 monoclonal coated on the T region. Results appear in 10 to 20 minutes in the form of a red line that develops on the strip. Whether the sample contains the SARS-CoV-2 antigen or not, the solution continues to migrate to encounter another reagent (an anti-mouse IgG antibody) that binds the remaining conjugates, thereby producing a red line on the region C.
Kit Content 1). Test device (individually packed in a foil pouch. 2). Extraction buffer vial. 3). Sterile swab. 4). Instruction for use.
Materials Provided: 1. Test Device 2. Sterilized Swab 3. Extraction Vial 4. Nozzle With Filter 5. Sample Extraction Buffer 6. Package Insert Materials Required but not Provided 1. Timer 2. Transfer pipette

Specimen Preparation: 1) Nasal Aspirate Fluids Add 10 drops (about 0.3 ml) of the nasal aspirate fluids into the extraction vial which contains 0.3 ml of the extraction buffer and mix well to be used as test sample. 2) Nasal Swabs Insert the swab into the extraction vial which contains 10 drops (about 0.3 ml) of the extraction buffer. Rotate the swab inside the vial using a circular motion to roll the side of the extraction tube so that liquid is expressed and reabsorbed from the swab. Remove the swab. The extracted solution will be used as test sample.
Test Procedure
Allow the test, specimen, extraction buffer to equilibrate to room temperature (15-30°C) prior to testing. 1. Remove the test device from the sealed foil pouch and use it as soon as possible. Best results will be obtained if the assay is performed immediately after opening the foil pouch. 2. Put the test device on a clean and level surface. 3. Shake the swab specimen in the extraction vial to well mix. 4. To run test, twist open the bottom screw cap of the extraction vial to expose dropper tip. Transfer 3 drops (~90μl) of the specimen to the sample well of the test device and make sure a colored liquid appearing in the detection window in 30 seconds. Replace cap cover on the extraction vial. 5. Start timer. Read the result at 10~20 minutes. Do not interpret the result after 20 minute.

Reading result

Cross Reaction
No cross reaction has been confirmed of the COVID-19 Ag Rapid Test Device with the following pathogens: ①Bacteria Acinetobacter baumannii, Bordetella pertussis, Branhamella catarrhalis, Candida albicans, Candida glabrata, Cardiobacterium hominis,Eikenella corrodens,Enterococcus faecalis, Enterococcus gallinarum.Escherichia coil, Group C streptococcus, Group G streptococcus, Haemophilus aphrophilus, Haemophilus influenzae, Haemophilus paraphrophilus, Klebsiella pneumoniae, Neisseria gonorrhoeae Peptococcus asaccharolyticus, Peptostreptococcus anaerobius, Proteus mirabilis, Proteus vulgaris, Pseudomonas aeruginosa, Serratia marcescens, Staphylococcus epidermidis, Streptococcus agalactiae(group B), Streptococcus mutans, Streptococcus pneumoniae, Streptococcus pyogenes(group A), Veillonella parvula ②Virus Influenza A,Influenza B, Adenovirus Type 1~8, 11, 19, 37, Coxsackie virus Type A16, B1~5, Cytomegalovirus, Echovirus Type 3, 6, 9, 11, 14, 18, 30, Enterovirus Type 71, HSV-1,Mumps virus, Tyep I simple herpes virus Parainfluenza virus Type 1~3, Poliovirus Type 1~3, Respiratory syncytial virus, Rhinovirus Type 1A, 13, 14, Type I simple herpes virus. ③Mycoplasma etc. No cross reaction with Chlamydia pneumoniae, Chlamydia psittaci, Chlamydia trachomatis, Mycoplasma pneumoniae.
 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!  Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters! Please check your E-mail!
Please check your E-mail!